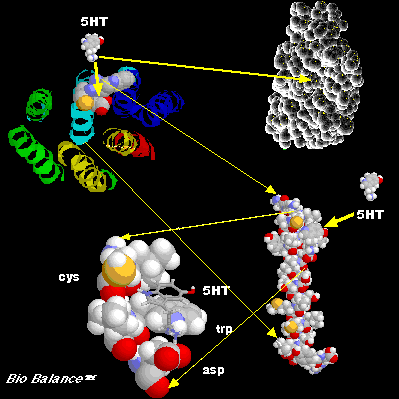
This image is a conception of how 5HT binds to the
5HT2A receptor. In the upper portion of the image are two views looking down on
the top of the receptor. The first view on the left is of the seven
transmembrane helices with the three important residues, cys, trp, and asp,
shown with spacefilling models. The 5HT agonist-ligand is also shown as a
spacefilling model approaching the putative binding region in the receptor.
The second view in the upper right is an image
produced from scanning the top of a spacefilling molecular model of the
receptor. This image suggests that there is a pocket where the ligand could
bind within this region.
The electrostatic attraction between the cationic
amine sidechain of the ligand and the anionic sidechain of the aspartate
contributes to the net electrostatic attraction of the ligand for the receptor
pocket.
Helix III is shown removed from the seven helices.
Within helix III there exists an ideal binding region for the 5HT ligand. This
region is further enlarged from helix III and shown in the lower left portion
of the image. The ring of the 5HT can interact with the ring of the trptophan
residue. In addition the cysteine residue can modulate the binding by being in
either an acid or base form. (this was work done over an eight year period with
Dr. Lester A. Rubenstein at Mt. Sinai School of Medicine, NYC).
*To see the electrostatic
potentials of the ligands with the two-states of the receptor click here
This image shows the two states (cysteine negative
and neutral) of the 5HT2A receptor with the 5HT bound to the three essential
residues in the third transmembrane domain (cys, trp and asp).
The coloring is of the electrostatic surface of the
two states with the negative or base form of the cysteine colored more red and
the neutral or acid form of the cysteine colored more blue.
The electrostatic attraction between the cationic
amine sidechain of the ligand and the anionic sidechain of the aspartate
contributes to the net electrostatic attraction of the ligand for the receptor
pocket.
It is the net difference in the electrostatic
energies between the acid and base forms of the receptor which corresponds to
the relative efficacies of several tryptamine molecules.
(See this link to the
graphics section)
Web Page: http://www.bio-balance.com